This post was originally published on this site
Here’s a question for everyone in Congress and the White House, the federal bureaucracy, and all my fellow members of the (ahem) “mainstream media.”
Now that Biogen
BIIB,
has got approval for its new Alzheimer’s drug—the first approved by the FDA in almost 20 years — how much should they be allowed to charge for it?
What’s the ethically correct figure? What will exempt them from charges of “greed” or “gouging?” What is “too much?”
The manufacturing cost per pill? The cost plus a small margin? What’s the number?
I bring this up because few things matter as much for the lives of senior citizens in America—and seniors in the rest of the world—than the development of new drugs. I have personally seen up close how Alzheimer’s destroys people, sometimes as young as their 50s and 60s. This has included close members of my family.
Some six million Americans already have this cruel, vicious and incurable disease, and a horrific 50 million around the world. That’s 50 million death sentences. Ten million more get it every year.
The drug industry developed five vaccines in one year for COVID-19, a disease associated with the deaths of 3.7 million people world-wide. Alzheimer’s treatments? Oh, one for every 20 years.
Let’s be honest: Many people subconsciously shrug off this issue on the principle that Alzheimer’s will only happen to someone else. Alas, many of the people reading this article are going to get this disease. For many more, your father, mother, wife or husband will get it. Or a sibling or close friend. At which point, the “hey, maybe we should have made this a bigger national priority” argument will strike, but too late to help.
This is the context worth bearing in mind when we hear about drugs getting approved—and, just as importantly, when we hear people demagoguing about “greedy” drug companies and “overpriced” drugs. It’s not just this new Alzheimer’s drug, either.
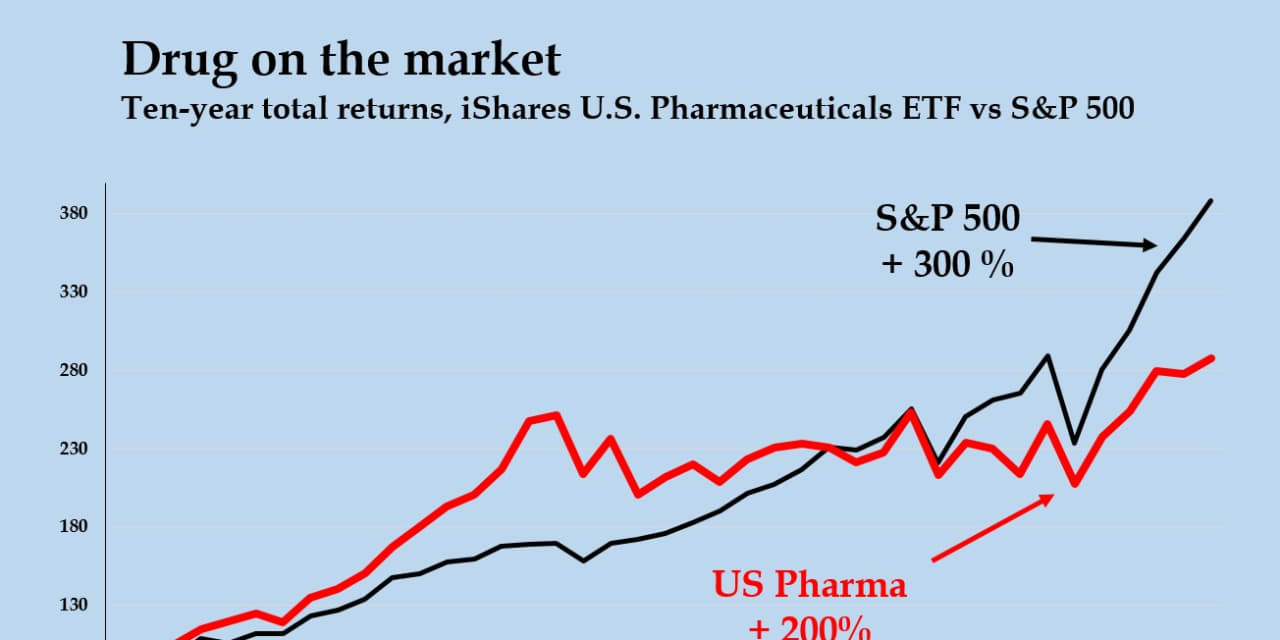
If “drug” companies are making out like bandits, someone really has to explain to me why it doesn’t show up in the…er…stock prices. In the past decade, investors the iShares U.S. Pharmaceuticals exchange-traded fund
IHE,
have made far lower returns than they could have done just by randomly picking a bunch of non-pharmaceutical stocks out of the newspaper. These companies have been worse investments than a simple S&P 500
SPX,
index fund like the SPDR S&P 500 ETF
SPY,
or the Invesco S&P 500 Equal Weight ETF
RSP,
Even Biogen itself had substantially underperformed the broader stock market index for a decade. Until Monday.
No, the industry returns aren’t terrible. But they are hardly the giant free oil well that critics sometimes suggest. I checked out the data from Dartmouth College finance professor Kenneth French, who has tracked stock market returns by industry going back decades. Going all the way back to the 1920s, according to his numbers, the pharmaceuticals industry barely cracks the top one-third of all industries by stock returns. So far this millennium, the total returns from major pharmaceutical companies have been about a third less than those from the S&P 500 index. Biotechs have done much better, but they were a much smaller sector. And even biotech has trailed the returns of alcohol and tobacco companies.
How are we going to get billions of dollars more capital invested in curing Alzheimer’s and diabetes and cancer when investors can make more money investing in making booze and cigarettes? This is upside down.
Meanwhile the regulation are all messed up and back to front as well. This headline says it all: “FDA Approves Alzheimer’s Drug Despite Fierce Debate Over Whether It Works.”
This is nuts. Maybe the new treatment makes things better, maybe it doesn’t. Apparently people aren’t certain. It’s hardly a surprise: The treatment is new, and the disease is complicated. But the first question to ask isn’t whether it makes things better, but whether it makes things worse. The current situation is catastrophic for anyone with the disease. People are dying. Lives are being destroyed.
Waiting to be sure treatment works would be like falling out of a plane with a parachute on, but hesitating to pull the cord because, well, it might not work. It suggests a basic misunderstanding of game theory. (Or common sense.)
It’s one thing to hesitate about approving a new drug for a nonfatal illness. You don’t want to make people sicker, or cause needless suffering or death. But with this kind of illness that really doesn’t apply.
Anyone who says “oh, no, we need to be really careful, and if need be delay these new treatments for years to make sure they are absolutely 100% safe” is speaking from a position of privilege. They should try saying that to someone watching a loved one be destroyed by the illness. It’s easy to play that card when it’s not happening to you, or someone you love.
A look through the Biogen’s public filings tells the sorry story. This new Alzheimer’s treatment entered Phase 3 trials in 2015—six years ago. Did I mention it took one year to get COVID vaccines to market?
Meanwhile, check out all the costs associated with developing this treatment. Biogen struck a collaboration deal with Eisai to work on this drug in 2014. Since then the two of them have spent a total of $1.1 billion on it. If it hadn’t been approved, that’s $1.1 billion down the drain.
I want more of these treatments. And I want them quickly, not every two decades. I want trillions pouring into this industry. And I know that lower returns, and more bureaucracy, aren’t the way to get them.
None of this, of course, means blind, passive or foolish naiveté about the drug industry or pricing. Drug manufacturers spend about $160 million a year on lobbying, and people involved in the business donated nearly $30 million a year to politicians running for office. Look through the proxy statements and you’ll see plenty of Big Pharma CEOs living off the fatta the lan (like lots of other CEOs). Yes, of course Medicare should have greater freedom to negotiate drug prices.
Few people know more about malfeasance and greed in the drug industry than investigative journalist Gerald Posner, who literally wrote the book on it — Pharma: Greed, Lies, and the Poisoning Of America.
“Most of the time I agree with your take,” he tells me. “FDA red tape bureaucracy is slow and costly and good R&D can cost a fortune, all with no guarantee of success. So risk takers, i.e. drug companies, should be rewarded with financial incentives.”
But, he adds, U.S. drug companies are already getting plenty of help. The U.S. has the longest patent protections of any major economy. He adds that big U.S. pharma companies get huge research support from taxpayers through the National Institute for Health.
“From 2010 through 2016, every one of the 210 drugs approved for sale by the FDA were completely or in part funded by the NIH,” he says. The fund came to more than $100 billion, he adds.
All good points. But I fear that as long as it’s more profitable to invest in cigarettes or booze than it is in curing Alzheimer’s, we will all be left with Plan B for our old age: Hoping and praying we don’t get Alzheimer’s.

