This post was originally published on this site
Pfizer CEO Albert Bourla revealed he personally received a COVID-19 vaccine booster recently and expects federal regulators to make booster shots more widely available in the United States.
“Likely, they will start moving the recommendations to earlier ages as they did in England and other places,” said Bourla. “I just received it.”
Bourla, 59, made the comments at the HTLH conference taking place this week in Boston. He said he got his booster shot in accordance with U.S. guidelines before traveling to Europe over the last two weeks.
Pfizer
PFE,
and vaccine-development partner BioNTech
BNTX,
initially sought to have booster shots of their COVID vaccine authorized for people 16 and over in the U.S. The Biden administration supported the move and raised expectations among many Americans that they would be able to receive a booster eight months after they had been jabbed a second time.
“‘It is not an easy discussion because hesitancy is never based on facts and data, which is the language that usually us, the scientists, speak; it’s in most of the cases fear. I have found in life in general there is only one emotion that is stronger than fear, and this is love.’”
But regulators and policy makers at the Food and Drug Administration and the Centers for Disease Control and Prevention did not think the existing data supported authorization of such wide distribution of boosters. Instead, U.S. regulators in September authorized a third shot of the Pfizer-BioNTech vaccine only for people 65 and over, and those who are at high risk of severe illness due to certain underlying medical conditions, or are otherwise facing higher risk of exposure because of the nature of their jobs.
The conflicting policy views on boosters coming from the White House and federal health officials created a lot of confusion in recent weeks. But Bourla thinks that, as more data are gathered, it will become more clear that boosters should be authorized widely among the U.S. population, he said. He pointed to data that health officials have accumulated in Israel, a country that experienced widespread vaccination a few months earlier than the U.S. and where Pfizer has an agreement to access public health data.
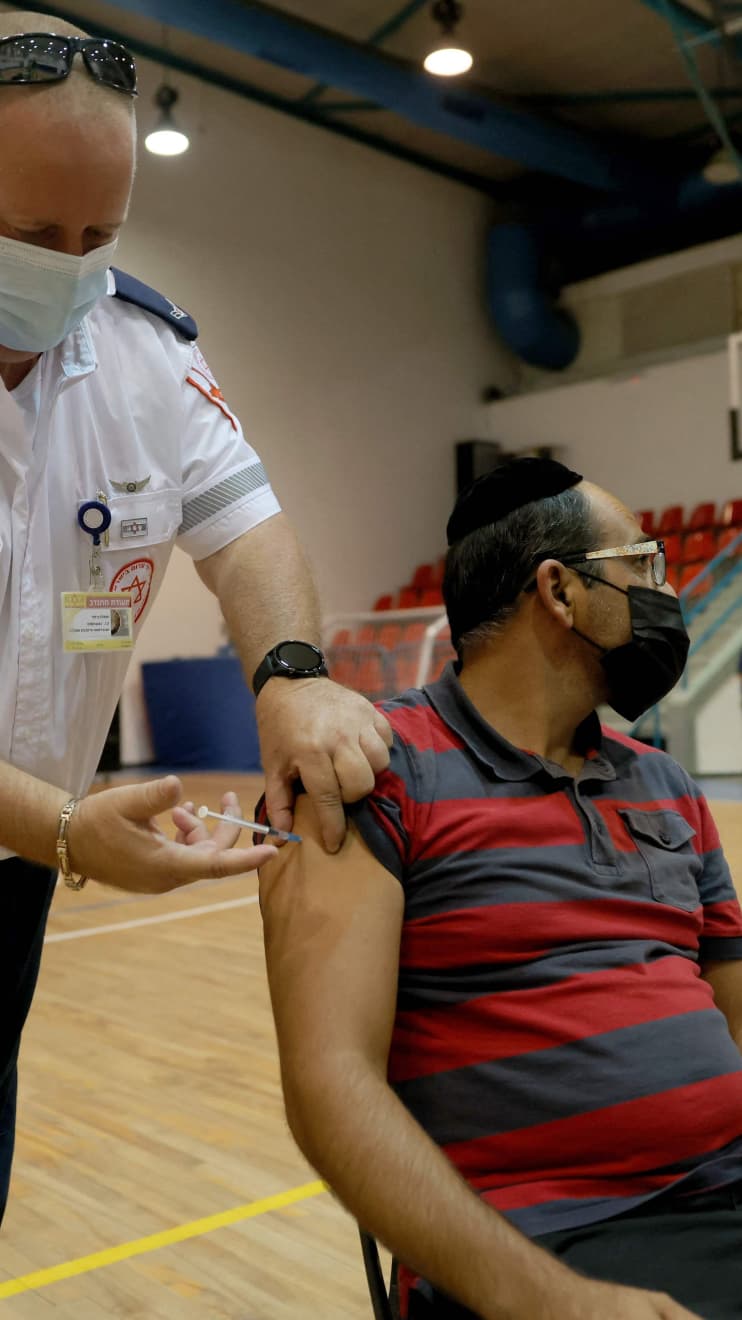
A paramedic with Israel’s Magen David Adom medical service administers a booster shot of the Pfizer-BioNTech vaccine in August.
ahmad gharabli/Agence France-Presse/Getty Images
“We did see in the beginning we had a waning of immunity that affects those who were six months” past their second shot, said Bourla, adding that Israeli health officials were not invited to present data to CDC officials as they were assessing boosters.
“In the beginning it was not hospitalizations, what was really going on was infections, but then in the seven months hospitalizations started and pretty soon followed deaths.”
See: COVID-19 vaccine booster shots are more complicated than they appear. Here’s why.
Bourla predicted U.S. health officials would ultimately follow paths blazed by their peers in countries including the U.K., where boosters are available for those over the age of 50, and Israel, which has been distributing boosters to people as young as 12.
The World Health Organization has opposed the distribution of boosters in wealthy countries out of concern boosters will diminish the supply of primary doses in poorer countries. Bourla said Pfizer, a U.S. based pharmaceutical behemoth, has invested heavily to make sure boosters will not cause vaccine inequity. He pointed to the 3 billion doses Pfizer and BioNTech are producing in 2021 and the 4 billion doses they plan to produce in 2022, with 1 billion doses being delivered to low- and middle-income countries this year.
Daily Coronavirus Update: U.S. may soon authorize boosters for all 3 in-use vaccines, as experts renew plea for unvaccinated to get their shots
Bourla very publicly waited in line until March to receive his first dose of the Pfizer-BioNTech vaccine. With the booster, he did not see a reason to wait because there was no public perception in the U.S. that people were unfairly getting access to boosters ahead of others, like earlier in the year when there was a shortage of vaccine supply for primary doses. Bourla did not elaborate on which criterion afforded him access to a booster under U.S. guidelines.
Bourla did, however, talk about how he has spoken to those who are hesitant to get a vaccine at a time when only 57% of all people in the U.S. are fully vaccinated (and 66.7% of those 12 and over), according to the CDC.
“It is not an easy discussion because hesitancy is never based on facts and data, which is the language that usually us, the scientists, speak; it’s in most of the cases fear,” Bourla said. “I have found in life in general there is only one emotion that is stronger than fear, and this is love.”
Bourla described how he tries to empathize with vaccine-hesitant people and understand the source of their fear while also making the point that their decisions will not only impact their own health, but the health of others, particularly those they love and spend time with frequently and closely.
“Try to conquer your fear by using the love from the people around you and listen, trust the millions of people who have done it,” Bourla said. “This is the best way right now to convince people, [but] it’s not going to be easy.”

