This post was originally published on this site
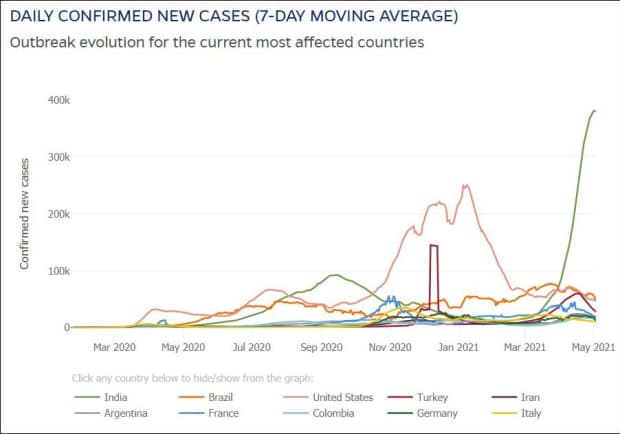
The global tally of confirmed cases of the coronavirus-borne illness COVID-19 climbed above 153.5 million on Tuesday, and India’s case tally rose above 20 million, after nearly doubling in three months.
India is now the second country with more than 20 million cases after the U.S., and its death toll has passed 220,000. The true numbers are expected to be way higher given that hospitals are overwhelmed, patients are dying in ambulances as they wait for ICU beds or oxygen that is in short supply, crematoria are turning people away and people are using parks and car parks for funeral pyres.
Infections have soared since February, a month after Prime Minister Narendra Modi boasted at the World Economic Forum of India’s success in containing the pandemic, as new and more contagious variants have swept across the nation of almost 1.4 billion people. Modi has been widely criticized for allowing superspreader events, including political rallies and a Hindu festival to take place, even as numbers were rising.
Don’t miss: Letter from India: ‘We live in mortal fear of COVID-19’
India’s health ministry reported 357,229 new cases on Tuesday and 3,449 deaths. But in an example of how those numbers are likely not reflecting the true count, the Associated Press reported that municipal records for Sunday showed 1,680 deaths in Delhi were treated according to protocols for handling COVID dead, but only 407 deaths were added to the official toll.
Dr. Ashish Jha, dean of Brown University’s School of Public Health in the U.S., told the AP he is concerned that Indian policymakers he has been in contact with believe things will improve in the next few days.
“I’ve been … trying to say to them, `If everything goes very well, things will be horrible for the next several weeks. And it may be much longer,’” he said.
There was positive news on the vaccine front in a report that the U.S. Food and Drug Administration is expected to grant emergency use authorization to the vaccine developed by Pfizer Inc. and German partner BioNTech SE for use in young people aged 12 to 15 by next week, a move that would greatly expand the base of people eligible for vaccination.
A person familiar with the process told the AP that the FDA is expected to authorize the vaccine for use in even younger children sometime in the fall. Results also are expected by the middle of this year from a U.S. study of Moderna’svaccine in 12- to 17-year-olds.
Younger people have made up a bigger share of new COVID cases since the vaccine program kicked into high gear and older people started to be at least partially vaccinated, and as outdoor activities, including sports and indoor dining resumed. Experts hope vastly expanding the program will help reduce the nation’s virus caseload and stop new variants from emerging.
The Centers for Disease Control and Prevention’s vaccine tracker is showing that as of 6.00 a.m. Eastern time Monday, 312.5 million doses had been delivered to states, 246.8 million doses had been administered, and 147.5 million people had received at least one shot, equal to 44.4% of the population.
A full 105.5 million people are fully vaccinated, equal to 31.8% of the population, meaning they have received two shots of the two-dose vaccines developed by Pfizer Inc.
PFE,
and German partner BioNTech SE
BNTX,
and Moderna Inc.
MRNA,
or one shot of the Johnson & Johnson
JNJ,
one-shot vaccine. The AstraZeneca
AZN,
AZN,
vaccine has not been authorized for use in the U.S.
Among Americans 65 years-and -older, 38 million people are fully vaccinated, equal to 69.7% of that group. More than 45 million people in that age bracket have received a first jab, covering 82.8% of that population.
See now: What we know about COVID vaccine side effects in women
In other news:
• Pfizer posted better-than-expected firs-quarter earnings on Tuesday, and raised its full-year guidance, as revenue expectations for its COVID-19 vaccine jumped 73%, MarketWatch’s Tomi Kilgore reported. Vaccine revenue tripled, to $4.89 billion from $1.61 billion, while oncology revenue rose 18% to $2.86 billion and internal medicine revenue grew 11% to $2.59 billion. For 2021, the company raised its guidance ranges for adjusted EPS to $3.55 to $3.65 from $3.10 to $3.20 and for revenue to $70.5 billion to $72.5 billion from $59.4 billion to $61.4 billion, as revenue expectations for the COVID-19 vaccine (BNT162b2) increased to $26 billion from $15 billion. The revenue projection for BNT162b2 includes guidance for 1.6 billion doses expected to be delivered this year.
• Moderna will double the size of its manufacturing space at its facility in Norwood, Mass., essentially allowing for a 50% increase in the production of its COVID-19 vaccine there, MarketWatch’s Jaimy Lee reported. Moderna now aims to produce between 800 million and 1 billion doses of its COVID-19 vaccine in 2021 and up to 3 billion doses in 2022. “We believe that this investment and expansion at our technology center will allow us to continue to optimize our mRNA products as we explore new pharmaceutical delivery forms such as prefilled syringes and lyophilized products,” Moderna CEO Stéphane Bancel said in a news release.
•Shares of microcap Precipio Inc.
PRPO,
a specialty diagnostics company, soared 65% Tuesday, adding to gains of more than 170% on Monday, after the company said its COVID-19 rapid antibody test is now available on Amazon.com’s
AMZN,
business platform. The 20-minute test, which was awarded emergency use authorization from the U.S. Food and Drug Administration in December, can only be purchased by qualified medical point-of-care providers. “Physicians and other medical facilities may now purchase these items directly from Amazon, and receive them within 2 business days,” the company said in a statement on Monday. The test can identify the presence of antibodies that have been developed from exposure to the coronavirus-borne illness, or from vaccination and show an immune response.
Read now: One prediction of returning to work post-COVID: ‘Unpredictable and potentially chaotic’
• A Canadian committee is recommending the use of the Johnson & Johnson vaccine for all Canadians 30 years of age and older, Canadian Broadcasting Corp. Radio-Canada reported, although it says individuals should weight the risk of highly rare but risky blood clots. Health Canada approved the J&J vaccine in March and updated its labeling last week to acknowledge the risk of a rare but serious blood-clotting condition connected to the shot. The same issue caused U.S. regulators to pause the use of the vaccine for two weeks, before experts deemed the benefits of the one-shot jab outweigh the risks.
• The two drug store chains that the federal government is using to expand its COVID vaccine program account for most wasted vaccine doses, according to a Kaiser Health News report. The CDC counted 182,874 wasted doses as of late March, of which CVS and Walgreens accounted for 21%, or nearly 128,500 wasted shots, the report said. The data suggests that the companies have wasted more doses than states, U.S. territories and federal agencies combined. Pfizer’s vaccine, which in December was the first to be deployed and initially required storage at ultracold temperatures, represented nearly 60% of tossed doses.
Latest tallies
The global tally for the coronavirus-borne illness rose above 153.6 million on Tuesday, according to data aggregated by Johns Hopkins University, while the death toll rose to 3.2 million. More than 90 million people have recovered from COVID, the data show.
The U.S. continues to lead the world in cases and deaths by wide margins, with 32.5 million cases and 577,571 deaths, or about a fifth of the worldwide tallies.
India is second with 20.3 million cases and third by fatalities at 222,408, according to its official figures.
Brazil is third with 14.8 million cases and second by fatalities at 408,622.
Mexico has the fourth-highest death toll at 217,345 and 2.3 million cases, or 15th highest tally.
The U.K. has 4.4 million cases and 127,799 deaths, the fifth-highest in the world and highest in Europe.
China, where the virus was first discovered late last year, has had 102,549 confirmed cases and 4,846 deaths, according to its official numbers, which are widely held to be massively underreported.
What’s the economy saying?
The U.S. trade deficit rose 5.6% in March to a record $74.4 billion, reflecting a seemingly insatiable appetite among Americans for consumer goods such as toys, clothes, cell phones and home furnishings as the economy gains speed, MarketWatch’s Jeffry Bartash reported.
The trade gap widened from $71.1 billion in February, the government said Tuesday. Economists polled by Dow Jones and The Wall Street Journal had forecast the deficit to total $74.8 billion.
Imports jumped 6.3% in March to $274.5 billion. Exports rose 6.6% to $200 billion.
The Dow Jones Industrial Average
DJIA,
and S&P 500
SPX,
were set to open lower in Tuesday trades.

