This post was originally published on this site
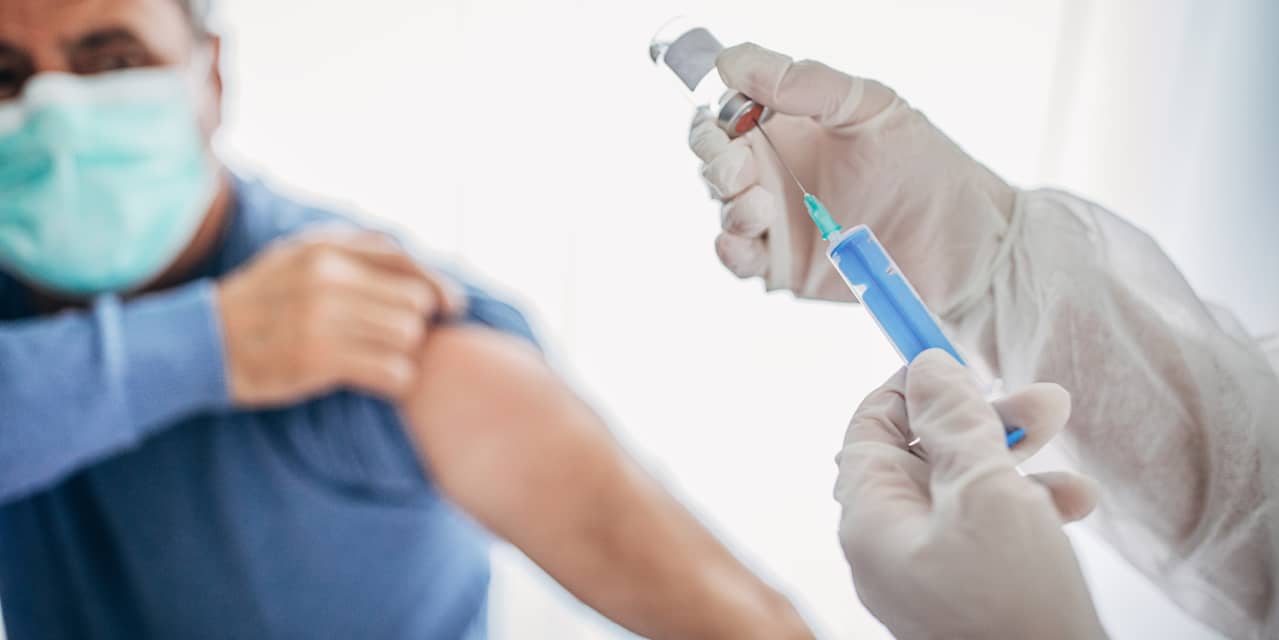
Dorothy Sauser-Monnig, of St. Paul, Minn., considers a COVID-19 vaccine her ticket to travel again. David Bakke, of Atlanta, said no to the flu vaccine in the past, but he got that shot this year and intends to get the coronavirus vaccine.
Carol Gee, of Atlanta, is a bit more cautious, but she hopes the COVID-19 vaccine will offer protection against the potentially serious complications the virus could cause for her and her husband, both of whom suffer from Type 2 diabetes.
Sauser-Monnig, Bakke and Gee, all over 50, are among the growing number of Americans who are expressing cautious enthusiasm for the coming wave of COVID-19 vaccines. The percentage of Americans who say they intend to get vaccinated is now over 80%—up from 51% in September — a new poll from ABC News/Ipsos reveals.
Among adults over 50, receptiveness toward vaccination is at 58%, with those over 65 citing the highest level of interest, according to the University of Michigan’s recent National Poll on Healthy Aging, which surveyed Americans age 50 to 80.
And while there are still pockets of resistance to the vaccine, particularly in communities of color, public health experts are optimistic that more of the population will come around to the idea of rolling up their sleeves to get the shot.
“The first vaccinations are likely going to be among health care workers, and I think it will be important for the general public to see these doctors and nurses lining up,” says Dr. Preeti Malani, chief health officer and professor of medicine at the University of Michigan. “Even though the vaccines were developed rather quickly, people will say, ‘If they think it’s safe, then it’s safe for me, too.’”
See: Here’s when most Americans will be able to get a COVID-19 vaccine
The first COVID-19 vaccine on the market, from Pfizer PFE, -0.92% and rolling out across the country this week, uses a new technology called mRNA, which consists of bits of genetic material from the virus that stimulate the immune system to make antibodies against it. Moderna’s MRNA, -2.62% mRNA vaccine is expected to be cleared by the Food and Drug Administration (FDA) soon. There are no other mRNA vaccines on the market, a novelty factor that makes some people uncomfortable.
Pfizer’s data from its Phase 3 trial shows its vaccine is 95% effective across demographic groups, with no major side effects reported.
When Next Avenue asked readers on Facebook FB, +0.70% for their opinions on getting a COVID-19 vaccine, several responded passionately against the idea. Some said the lightning-fast development process, called Operation Warp Speed, made them afraid they would be “lab rats” for untested products.
“Not a chance I’m putting this poison in my body,” said one. Another griped that the Centers for Disease Control and Prevention (CDC) and FDA had “lost all credibility” under President Donald Trump.
Will Dr. Fauci get the vaccine?
Bakke, 53, says he’s relying on medical experts like Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases — not politicians — to offer advice on the safety and efficacy of COVID-19 vaccines. Fauci has said he would get the vaccine once it’s approved and that he trusts the transparent nature of the FDA’s data review process.
“This whole pandemic and even vaccines have been so totally politicized that it’s really scary to me. The pandemic is real…and folks need to understand that it’s in their best interests to get vaccinated,” says Bakke, a content strategist for National Air Warehouse. Bakke says he’ll request a vaccine as soon as it becomes available to him.
Gee, 70, is certain she’ll be vaccinated eventually, but she will wait until enough older Americans with health conditions such as diabetes have received the shot, so she can be sure they’re not experiencing dangerous side effects.
“I’m African-American, and I need to see how it’s affecting African-American people,” says Gee, a retired administrator at Emory University. Her husband also suffers from congestive heart failure and was recently treated for prostate cancer.
“I have my annual wellness visit next month and will ask my doctor about the vaccines,” Gee says. “I’ll ask my husband’s cardiologist and oncologist, so I can get as much information as possible to make the right decision for both of us.”
Gee isn’t alone in her desire to gather more information before getting vaccinated. The National Poll on Healthy Aging revealed that only 7% of Black people age 50 to 80 surveyed want to get a COVID-19 vaccine as soon as possible, compared with 24% of white people that age.
Distrust among communities of color
Vaccine reluctance in minority populations is nothing new, says Dr. David Carlisle, CEO of the Charles R. Drew University of Medicine and Science in Los Angeles, a historically Black graduate school.
Much of that, he says, dates back to the infamous Tuskegee experiment, a 40-year syphilis study that ended in 1972 amid outrage over deaths among African-American participants who were denied proven treatments so researchers could study the natural progression of the disease.
“For older people, that experiment was personal,” Carlisle says. “Then add to that a legacy of mistrust. Some of these individuals were alive when we were trying to desegregate hospitals. No wonder they’re reticent to jump into a new health-care technology with both feet.”
Carlisle has been following the COVID-19 vaccines closely and says he’s confident the developers have done what’s necessary to prove safety and efficacy, including enrolling minorities in the clinical trials.
That sentiment was echoed by Dr. Ramin Ahmadi, a former assistant professor of medicine at Yale who is now chief medical officer for GMED Global, a health care consulting firm.
“Yes, they developed these vaccines faster than we were able to in the past. But I don’t think they cut any corners,” Ahmadi says. “I will be among the first people in line to get a vaccine.”
Also read: I’m 55, tired of ‘soul-crushing jobs,’ have $1 million invested poorly — can I retire now?
Sauser-Monnig, a retired elementary school teacher, says as soon as she and her husband get their COVID-19 vaccines, they plan to fly to France once every few months to see their daughter and her family who live there, and to explore parts of the U.S. and Canada they had planned to drive to before the pandemic shutdowns.
“We have a little trailer and we love road trips,” she says. “We want to hit the road.”
Arlene Weintraub is a science journalist and author who has contributed to Forbes.com, the New York Times, U.S. News & World Report, Cure, Fierce Markets and other media outlets. She was previously a senior writer based out of the New York City headquarters of BusinessWeek, where she wrote hundreds of articles that explored the science and business of health. She is the author of “Heal: The Vital Role of Dogs in the Search for Cancer Cures” and “Selling the Fountain of Youth. ”
This article is reprinted by permission from NextAvenue.org, © 2020 Twin Cities Public Television, Inc. All rights reserved.
More from Next Avenue:

